Swedish Medical Report: Traditional Cancer Drugs of Little to No Value - August 2023
2 out of 3 cancer drugs in widespread use show little to no benefit to quality of life or longevity, ZERO eradicate cancer.
Fenbendazole Can Cure Cancer presents Case Reports of people who have treated their own cancers along with other articles to help understand how fenbendazole works. Previous articles covering other cancers are in the Archives link. This article presents a bombshell of an new article by Swedish researchers demonstrating that many cancer drugs in use are useless in terms of extending life and/or enhancing the quality of life of the cancer patients that receive them. If you or loved ones are currently on chemotherapy, consider incorporating the following information in your library of knowledge when making informed choices. Sharing this article with your doctor may be a good idea as well.
Scientists continue to develop new treatments for cancer patients, not just in the US, but all over the world. However, a new study in the journal Clinical Drug Investigation finds that many of these drug (chemotherapy) treatments, are completely ineffective, failing to improve the quality of life or extend the lifespans of cancer patients. Not a big surprise to longtime readers of this Substack. Recall that we recently presented a peer-reviewed paper where cancer scientists called for replacing the current standard-of-care brain cancer drug (vincristine) with mebendazole, based on efficacy concerns.
Scientists at the University of Gothenburg in Sweden found that approximately two out of three cancer drugs prove to be useless for patients after examining the eventual outcome on patients in terms of longevity and quality of life measures.
Think about that, current cancer treatments considered “state-of-the-art” are basically useless according to this study, just published August 2023!
The details of the study are in the original article referenced below but here are the highlights. The Swedish team examined 22 cancer drugs approved for use in Sweden over the last 10 years, examining other studies that tested these drugs’ ability to improve quality of life or extend lifespan. The names of the specific drugs studied are listed below. These studies followed the drug’s performance for an average of 6.6 years after the drug was released for use.
Cancer Drugs studied in Chauca Strand et al., (2023) and their intended uses
Everolimus
Patients with advanced RCC, following VEGFR-targeted therapy treatment.Nilotinib®
Newly diagnosed patients with Ph+ CMLAxitinib
Patients with advanced RCC after failure of prior treatments with sunitinib or cytokines.Brentuximab or Vedotin
Patients with recurrent or refractory CD30+ Hodgkin’s Lymphoma; following ASCT or following at least two prior therapies when ASCT or chemotherapy is not a treatment option or will be doing a ASCT (used as neo-adjuvant treatment).Bosutinib
Patients with Ph+ CML in chronic, accelerated or blast phase previously treated with one or more TKIs and for whom imatinib, nilotinib and dasatinib are not considered appropriate treatment optionDabrafenib
Patients with unresectable or metastatic melanoma with a BRAF V600 mutationCabozantinib
Patients with progressive, unresectable locally advanced or metastatic medullary thyroid carcinoma.Olaparib
Maintenance treatment of patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer with BRCA-mutations who are in response to platinum-based chemotherapy.Idelalisib
Patients with follicular lymphoma that is refractory to two previous treatmentsPonatinib 1
Patients with CML in chronic, accelerated or blast phase, who are resistant to dasatinib or nilotinib; or who are intolerant to dasatinib or nilotinib and for whom subsequent treatment with imatinib is not appropriate; or who have a T315I mutationPonatinib 2
Patients with Ph+ Acute lymphocytic leukemia who are resistant to dasatiniband for whom subsequent treatment with imatinib is not appropriate; or who have T315I mutation.Ceretinib
Patients with advanced ALK+ non-small cell lung cancer who previously have been treated with crizotinib.Palbociclib
Patients with locally advanced or metastatic HR+, HER2-breast cancer (in combination with an aromatase inhibitor).Osimertinib
Patients with locally advanced or metastatic non-small cell lung cancer with EGFR T790M-mutation.Alectinib 1
Patients with ALK+ advanced non-small cell lung cancer who have been previously treated with crizotinib.Fulvestrant
Patients with ER+ locally advanced or metastatic breast cancer in postmenopausal women not previously treated with endocrine therapyRibociclib
In combination with an aromatase inhibitor as initial endocrine-based therapy for postmenopausal women with locally advanced or metastatic HR+, HER-breast cancerAlectinib 2
As first-line treatment for patients with ALK+ advanced, non-small cell lung cancer.Lorlatinib
Patients with ALK+ advanced non-small cell lung cancer whose disease has progressed after treatment with alectinib or certinib as first ALK tyrosine kinase inhibitor therapy; or crizotonib and at least another ALK TKI.Niraparib
As maintenance treatment for patients with platinum-sensitive relapsed high-grade serous epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response after platinum-based chemotherapy.Venetoclax
In combination with obinutuzumab for the treatment of adults with previously untreated CLL.Larotrectinib
Treatment of patients with solid tumors with NTRK-gene fusion with locally advanced or metastatic disease or where surgical resection is likely to result in severe morbidity or who have no satisfactory treatment options.
Abbreviations: ALK+, anaplastic lymphoma kinase-positive; ASCT, autologous stem cell transplantation; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; HR+, hormone-receptor positive; HER2, humanepidermal growth factor receptor 2; NTRK, Neurotrophic tyrosine receptor kinase;OS, overall survival; PFS, progression-free survival;Ph+, Philadelphia chromosome-positive;QoL, quality of life;RCC, renal cell carcinoma; TKI, tyrosine-kinase inhibitor
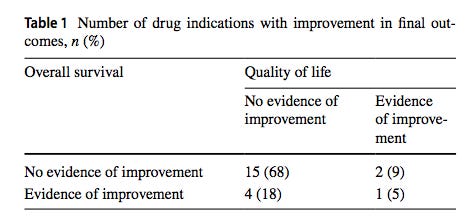
They found that only seven of the 22 drugs had at least one study which showed a clear benefit for cancer patients. Randomized controlled trials on the other 15 failed to show any measurable benefits for those with cancer. Only one, one!, out of the 22 drugs in the study showed an ability to both improve the quality of life and extend lifespans for patients. From Strand et al. (2023), “one drug, osimertinib, was found to improve both OS (longevity) and QoL (quality of life) for the indication of advanced NSCLC with EGFR T790M mutations. The improvements in median OS for these ranged from 2 to 13 months (n = 5).”
from Chauca Strand et al. (2023). Table shows the raw number of drugs in the category and the percentage that number represents in the parentheses.
83% of the cancer drugs tested (18 out of the 22) did not
significantly increase or decrease longevity or quality of life.
A deep dive into the meat of the paper indicates that the situation regarding the efficacy of these cancer drugs is even worse than the above headline reveals. Rather than the majority of the drugs simply being useless, some of them actually make things worse with respect to longevity and quality of life! Two drugs axitinib and “everolimus, the pooled effect estimate showed a significant negative impact on OS (longevity)”. Furthermore, the authors state that 83% or 18 of the 22 drugs tested did not significantly increase or decrease longevity or quality of life. The authors did not indicate the direction of the trend. We can only speculate as to whether the trend was toward helping the patient or hurting the patient!
Usually when universities release ground-breaking or controversial findings like these they have a PR department to accept the praise or deflect the criticism. In a release by the University of Gothenburg, the lead author Gabriella Chauca Strand defends her work, “We have shown that the majority of the drugs launched with limited evidence still lack clear evidence of how they actually affect survival and quality of life in patients.”
“The lack of confirmatory evidence for important patient outcomes is problematic and creates uncertainty about how these drugs actually contribute to meaningful patient benefit, and ultimately how effectively resources are being used within healthcare,” Strand also said.
The findings are published in the scientific journal Clinical Drug Investigation, the reference is below.
So this paper is from Sweden, how similar is this situation to that in the US? Very similar, if not identical. But don’t hold your breath waiting for a US research institution to ever publish findings like these as that would amount to biting the hand that feeds it. Many of the drugs used for cancer treatment improve longevity by 1%, 2%, 3% compared to controls, if at all, and as we’ve seen above, some of these drugs are linked to negative outcomes in longevity and quality of life, which means they disrupt and shorten the patient’s life. And at what cost in terms of human suffering and financial hardship? Read the disclosure information on any oncological drug for an eye-opening education of what passes as “useful” or “successful” in modern oncology.
One feature that is very useful on the Substack platform is the Comments function. Recently an obviously highly-educated and insightful reader made the comment that “modern oncology is at the leech and blood-letting stage of development compared to where fenbendazole is with respect to successfully dealing with cancer.” Let that sink in for a minute. The Case Reports presented here all deal with fenbendazole eradicating cancer. No where in the Swedish study was the word “cure” or “eradication” mentioned in conjunction with any of the cancer drugs studied. The notion of a cure is a foreign concept in “modern” oncology. Fortunately, we have fenbendazole!
This week, August 15, 2023, YouTube announced that it was starting a mass takedown of videos promoting “harmful” or “ineffective” cures from its platform, or videos that discourage anything other than traditional approaches to cancer treatment, see The Verge. I’m sure the intended targets were never considered to be the very drugs that the pharmaceutical companies promote online, on TV and in person which in turn pay the censors at YouTube to promote such nonsense. Talk about bad timing and shooting yourself in the foot on YouTube’s part! Based on the Chauca Strand et al. (2023) results proving that Big Pharma cancer drugs are junk, one would expect quite a housecleaning of Big Pharma drugs identified above from the YouTube platform.
Instead look for effective, safe, inexpensive, OTC, readily available cancer eradicating medicines like fenbendazole and ivermectin to be tarred-and-feathered and run off the platform.
Finally, how long until the Chauca Strand et al. (2023) paper is retracted? Our guess is by Sept 1, 2023. Not because any of the analyses or science is wrong, but because it’s bad for business.
A big tack (thanks in Swedish) to Mark, a loyal reader of the Substack, for alerting us to the Swedish paper!
References
Chauca Strand, G., Johansson, N., Jakobsson, N. et al. Cancer Drugs Reimbursed with Limited Evidence on Overall Survival and Quality of Life: Do Follow-Up Studies Confirm Patient Benefits?. Clinical Drug Investigation (2023). https://doi.org/10.1007/s40261-023-01285-4
Porter, Jon (2023). YouTube starts mass takedowns of videos promoting ‘harmful or ineffective’. The platform will also take action against videos that discourage people from seeking professional medical treatment as it sets out its health policies going forward. The Verge, August 15, 2023.
Fenbendazole vs. Mebendazole vs. Albendazole vs. Flubendazole: The benzimidazoles are very similar chemically and they have very similar mechanisms of action with respect to disrupting microtubule function, specifically defined as binding to the colchicine-sensitive site of the beta subunit of helminithic (parasite) tubulin thereby disrupting binding of that beta unit with the alpha unit of tubulin which blocks intracellular transport and glucose absorption (Guerini et al., 2019). If someone asks you how fenbendazole kills the cancer cells, the answer is in italics in the previous sentence.
The class of drugs known as benzimidazoles includes fenbendazole, mebendazole, albendazole and flubendazole. Mebendazole is the form that is approved for human use while fenbendazole is approved for veterinary use. The main difference is the cost. Mebendazole is expensive ~$555 per 100 mg pill, while fenbendazole is inexpensive ~48 cents per 222 mg free powder dose (Williams, 2019). As you may recall, albendazole is the form used to treat intestinal parasites in India and these cost 2 cents per pill. FYI, to illustrate how Americans are screwed by Big Pharma, two pills of mebendazole cost just $4 in the UK, 27 cents per 100 mg pill in India and $555 per 100 mg pill in the US.
While most of the pre-clinical research uses mebendazole, probably because it is the FDA-approved-for-humans form of fenbendazole, virtually all of the self-treating clinical reports involve the use of fenbendazole. Because the preclinical cancer studies use mebendazole (ironically the human form of fenbendazole) and humans self-treat their cancers with fenbendazole (the animal form of mebendazole) it is very reasonable to assume that mebendazole and fenbendazole are functional equivalents with respect to cancer. It would be helpful if future pre-clinical and clinical investigations simply used fenbendazole as a practical matter. For the purposes of this Substack, fenbendazole, mebendazole and albendazole are used interchangably.
Where to get fenbendazole
In our experience and the experiences of those that write in, it appears that the three readily available brands of fenbendazole (Panacur-C, FenBen Labs, Happy Healing Labs) are equally effective. Panacur-C can be obtained locally in pet stores, while they all can be obtained from Amazon.
If you would like to report your experiences with fenbendazole you can do so privately by email fenbendazole77@gmail.com or more publically in the Comments section in any of the articles. Also, if you know of people who’ve tried fenbendazole, and it didn’t work, we’d be especially interested in hearing from you now. Understanding the conditions and factors that enhance or impede the success of fenbendazole in treating cancer are valuable. As an example, we recently learned that pairing fenbendazole with oleic acid (olive oil has high oleic acid content) greatly enhances the bioavailability of fenbendazole (Liu et al., 2012). As a practical application of this information taking fenbendazole with a tablespoon of olive oil may result in better outcomes.
Disclaimer:
Statements on this website have not been evaluated by the Food and Drug Administration. The contents of this website is for educational and informational purposes only and is not intended to be a substitute for professional medical advice, diagnosis or treatment. This website does not provide any kind of health or medical advice of any kind. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. The case reports presented reflect the real-life experiences and opinions of other readers or users of the website. The experiences of those readers or users are personal to those particular readers/users and may not necessarily be representative of all readers/users. We do not claim, and you should not assume, that all other readers/users will have the same experiences. Do you own research, consult with relevant medical professionals before attempting to self-treat for any condition.